Philips iE33
Call to configure, special pricing available 317-759-9210
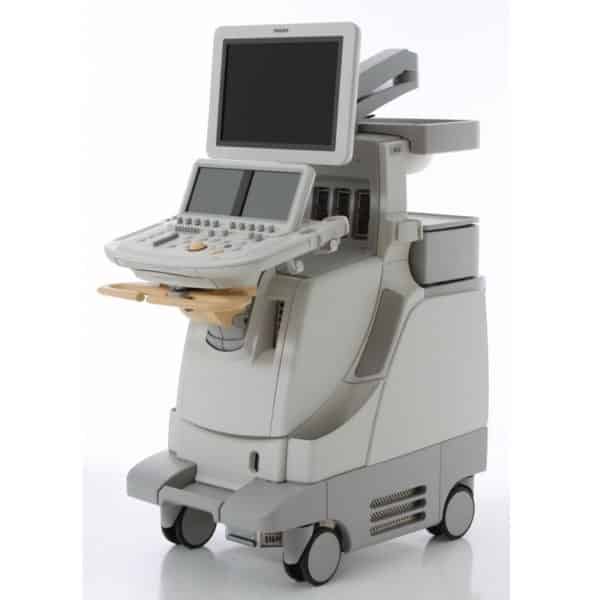
The refurbished Philips iE33 cardiovascular ultrasound machine, launched in 2004, is one of the most popular ultrasound machines in demand due to it being the cardiovascular gold standard for over a decade. In the updated 2010 model, the iE33 xMatrix, it is equipped with xMatrix transducers with thousands of elements each and is capable of viewing images in 2 planes in real time as well as 4D capabilities that do not require a mechanical probe.
The Philips iE33 xMatrix can enable QLAB quantification and a multitude of various cardiovascular specific software enhancements that will add diagnostic confidence to standard vascular and cardiac exams. The Epiq 7 is the replacement to the iE33. Those looking for similar features and power at the premium level with a focus on radiology or OB/GYN, buyers chose the Philips iU22, which looks identical to the iE33 except it has one touchscreen, whereas the iE33 has two.